Pharmacovigilance Writing and Consulting Support
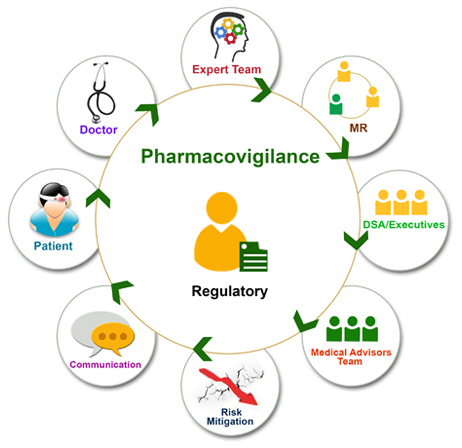
The safety check during the clinical trial is of extreme importance; however it is similarly critical when the drug is in the market. Performing pharmacovigilance and submitting the desired documents assuring the continuous safety is the shoulder responsibility of any drug/device company. We understand the patient’s concern and make sure the process is handled carefully right from the reporting of cases till the submission of aggregate reports. We keep the dates for submission of documents for which the regular updates are required in our calendar and remind our clients for such submissions to avoid any last minute hassle.
We also provide consulting services to any stage during the whole process of pharmacovigilance.