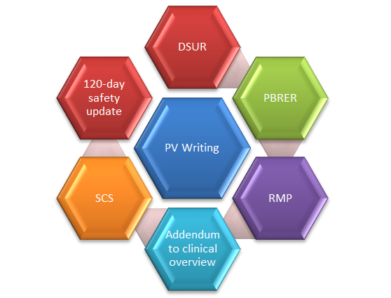
As we all are aware that pharmacovigilance (PV) is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other possible drug-related problems; however the concerns of pharmacovigilance (PV) are increasing nowadays for herbals, blood products, biological, medical devices, vaccines and traditional medicines too. These concerns include lack of efficacy reports, substandard medicines, medication errors, case reports of acute and chronic poisoning, assessment of drug-related mortality, abuse and misuse of medicines, ...
Read more...